April 8, 2020 feature
Massive generation of metastable bulk nanobubbles in water by external electric fields
Nanobubbles can exist on solid surfaces or in bulk liquids as nanoscopic gaseous domains. The phenomenon has attracted substantial attention due to the long-time (meta)stability and potential for practical applications. In a new report, Mohammad Reza Ghaani and a team of researchers in chemistry and bioprocess engineering in Ireland and Canada used a novel approach to explore the surface of electrostatic nanobubble (NB) formation. They observed the stability of the constructs by applying external electric fields in gas-liquid systems to observe massive gas uptake into the liquid in nanobubble form. During a period of time lasting months, the gas solubility enhanced from 2.5 fold for oxygen to 30-fold for methane, based on Henry's Law values for gas solubility—i.e., the more hydrophobic the gas, the greater the intake. Using molecular dynamics solutions, Ghaani et al. revealed the origin of NB's movement to result from dielectrophoresis, while the substantial stability of NB arose from surface-polarization interactions. The work is now published on Science Advances.
Nanobubbles are nanoscopic gaseous forms that can exist on solid surfaces or in bulk liquids. Bulk NBs can be present in most aqueous solutions due to constant agitation and cosmic radiation – attracting significant attention for applications in nanoscopic cleaning, to control boundary slip in microfluidics, wastewater treatment, heterocoagulation and medicine. Scientists credit the long-lived presence of NB's to negative-charge build-up at the bubble/liquid interface and a strong electron affinity at the surface. Independent of the NB diameter, the mutual repulsion between NBs in water are large enough to prevent coalescence and slow the rise of buoyancy. Scientists can regulate the size of NBs in the presence of surface-active agents and use resulting coated bubbles as ultra-sound contrast agents or for targeted drug delivery.
In this work, Ghaani et al. tackled fundamental factors governing the NB's pH-, ionic- and magnetic field-sensitive nature, including surface electrostatics. They aimed to determine if externally applied electric fields could manipulate, dictate, control and enhance NB formation. If such external forces had an effect, they investigated their energy cost and electro-induced alterations. When the team applied low electric energy, they observed massive and rapid enhancement of metastable NB gas accommodation in water. The scientists investigated if the first-in-study results for NB generation occurred in the bulk liquid or at the liquid interface and identified the phenomenon to be due to bulk NBs using a bulk-probing NB detection/diagnostic tool.
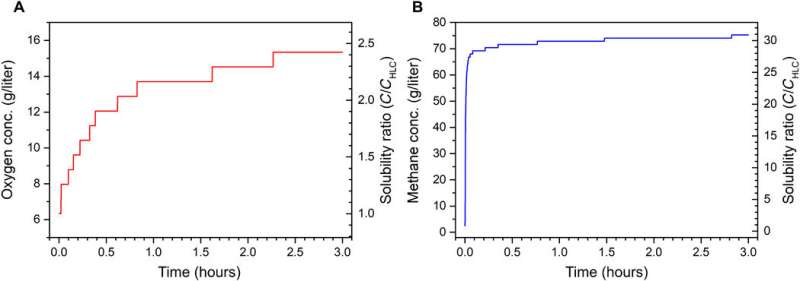
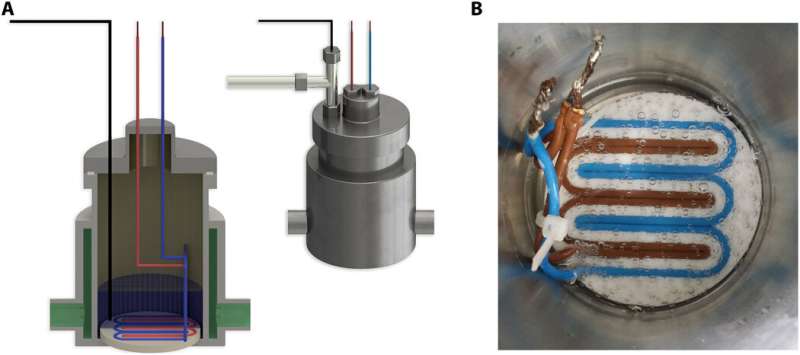
The team initially placed deionized water in a pressure vessel and fed pure gas to ~90 bar, closed the vessel and regulated the system's temperature. When the setup reached Henry's Law gas-solubility level within two hours, they activated an external sustained static electric field inside the liquid water using a 60 V direct current (DC) source. Within three hours or less, they achieved greatly elevated gas uptake plateau in the water and noted a flux of gas molecules form the bulk gas phase in the liquid during NB formation, causing the pressure to drop. Comparatively, the energy required to form NBs using electric fields were extremely low and pointed to extraordinarily high levels of energy efficiency.
For example, the energy required to form NBs equalled 0.3 W hour/m3; much lower than that in advanced systems such as wastewater industries (~40 W hour/m3). Furthermore, while wastewater aeration typically allowed ~1 to 2 mg/liter of dissolved oxygen, the team achieved ~25 to 35 mg/liter of dissolved oxygen with NBs that were metastable for months. Using nonequilibrium molecular dynamics (NEMD) Ghaani et al. then explored the underlying molecular mechanisms behind the startling gas accommodation increase observed experimentally in water. It appeared that the more hydrophobic the gas, the more accentuated the electric field effect to amplify the massive increase in the capability to form bulk NBs. The results also suggested that NB formation maybe kinetically dominated.
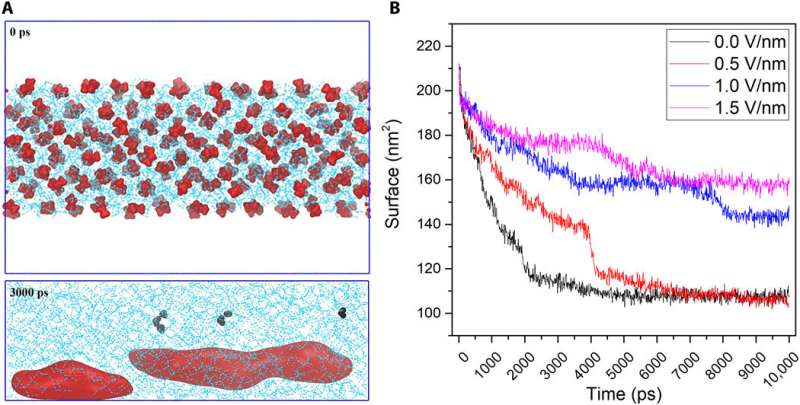
The team next ran NEMD (nonequilibrium molecular dynamics) simulations for both propane and methane in water and observed similar results for both gases. During the simulation, Ghaani et al. applied external fields of much greater intensity than those used for the experiments to observe credible results with minimal signal-to-noise ratio, for more than million-atom NEMD, spanning tens of nanoseconds. The more intense fields promoted NB formation readily with higher surface area in the simulation.
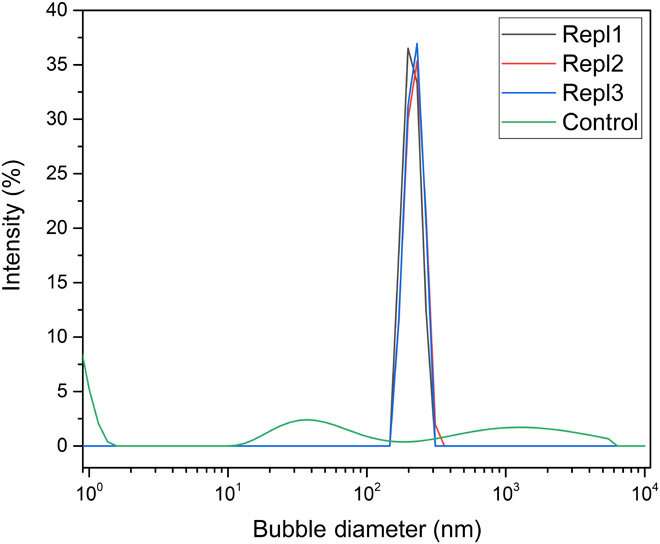
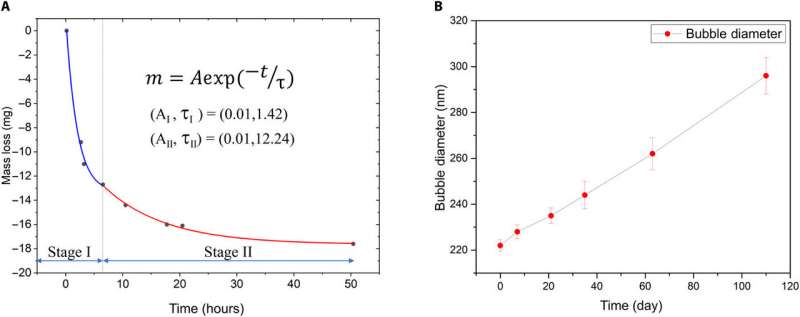
Since the long-lived NB stability is well known, the team studied the metastability of NBs after field removal and exposure to ambient pressure. To understand if NBs are localized at the surface or distributed in bulk, the team used dispersive light scattering (DLS) as a bulk probing method and detected NBs throughout the bulk liquid. However, the scientists also noted unusually transient micro-to-macro-sized bubbles at the polytetrafluoroethylene (PTFE) surface in the system born of nano- to micron-scale bubble nucleation after applying an electric field. Ghaani et al. observed the excess oxygen water/gas localized bubbles to destabilize mechanically within six hours, while limited bulk-bubble loss occurred after six to 50 hours. After a period of four months, the remaining NBs enlarged in size as measured with DLS (dispersive light scattering).
In this way, Mohammad Reza Ghaani and colleagues observed first-in-study evidence of bulk-NB formation with greater enhancement for more hydrophobic gases. The discovery will have a large impact in fermentation, brewing and wastewater treatment industries. The team propose further work to understand the mechanisms behind the kinetics of NB generation as well as NB stabilization thereafter. The research team realized "nanoporous liquids" in this work due to the presence of porous or "holey" liquids with gas NBs in a simple and facile manner.
More information: Mohammad Reza Ghaani et al. Massive generation of metastable bulk nanobubbles in water by external electric fields, Science Advances (2020). DOI: 10.1126/sciadv.aaz0094
Nicola Giri et al. Liquids with permanent porosity, Nature (2015). DOI: 10.1038/nature16072
M. N. Shneider et al. Cavitation in dielectric fluid in inhomogeneous pulsed electric field, Journal of Applied Physics (2013). DOI: 10.1063/1.4840935
Journal information: Science Advances , Nature , Journal of Applied Physics
© 2020 Science X Network