July 16, 2018 report
Making solar hydrogen generation more efficient in microgravity
An international team of researchers has found a way to make solar hydrogen generation more efficient in microgravity environments. In their paper published in the journal Nature Communications, the group describes what they learned from experiments with a photoelectrochemical cell falling in a drop tower.
In order to go very far in space, future astronauts will need some means for creating their own air and fuel—carrying enough of any of them for very long trips would prove impractical. Currently, astronauts aboard the ISS generate oxygen using a two-stage process. The first stage involves generating electricity using solar cells. In the second stage, the electricity is used to carry out an electrolysis technique with water. The researchers note that this process works, but it is inefficient. In this new effort, their goal was to improve the efficiency of the electrolysis technique used.
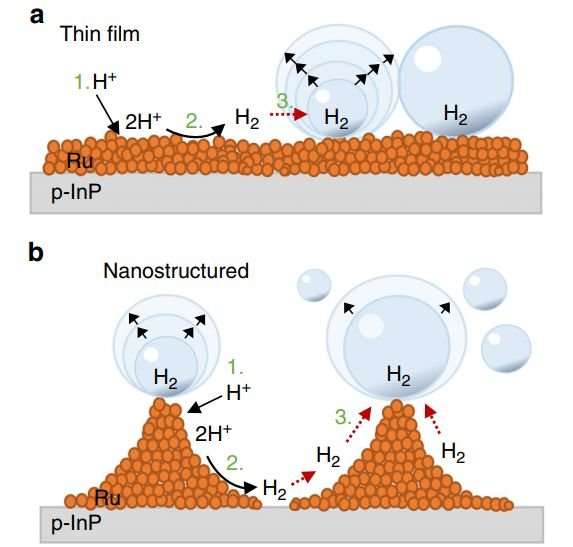
The researchers explain that the current process involves using an electrode made of a semiconductor that is light absorbing: Typically, a p-type indium phosphide. The electrode is then coated with a thin layer of a rhodium catalyst. As has been noted in the past, the inefficiency lies in the problem of hydrogen bubbles adhering to the surface of the electrodes, rather than bobbling up off them (due to buoyancy) as occurs on Earth. To get them to bobble up in a microgravity environment, the researchers changed the texture of the electrode. Rather than the normal flat surface, the team forced the rhodium into peaks and valleys, with the distance between them too far for the hydrogen bubbles to sit in. That meant they had to sit on the peaks, which left less contact between the bubbles and the surface.
To test their idea, the researchers created capsules containing their apparatus and dropped them 120 meters down the Bremen Drop Tower in Germany. They note that each drop occurred over approximately 9.3 seconds—enough time for their device to produce hydrogen gas.
The researchers found that their change to the surface of the electrode resulted in the production of hydrogen gas at the same rates as devices in normal gravity. They acknowledge that more work needs to be done, but suggest their approach looks promising.
More information: Katharina Brinkert et al. Efficient solar hydrogen generation in microgravity environment, Nature Communications (2018). DOI: 10.1038/s41467-018-04844-y
Abstract
Long-term space missions require extra-terrestrial production of storable, renewable energy. Hydrogen is ascribed a crucial role for transportation, electrical power and oxygen generation. We demonstrate in a series of drop tower experiments that efficient direct hydrogen production can be realized photoelectrochemically in microgravity environment, providing an alternative route to existing life support technologies for space travel. The photoelectrochemical cell consists of an integrated catalyst-functionalized semiconductor system that generates hydrogen with current densities >15 mA/cm2 in the absence of buoyancy. Conditions are described adverting the resulting formation of ion transport blocking froth layers on the photoelectrodes. The current limiting factors were overcome by controlling the micro- and nanotopography of the Rh electrocatalyst using shadow nanosphere lithography. The behaviour of the applied system in terrestrial and microgravity environment is simulated using a kinetic transport model. Differences observed for varied catalyst topography are elucidated, enabling future photoelectrode designs for use in reduced gravity environments.
Journal information: Nature Communications
© 2018 Phys.org




















